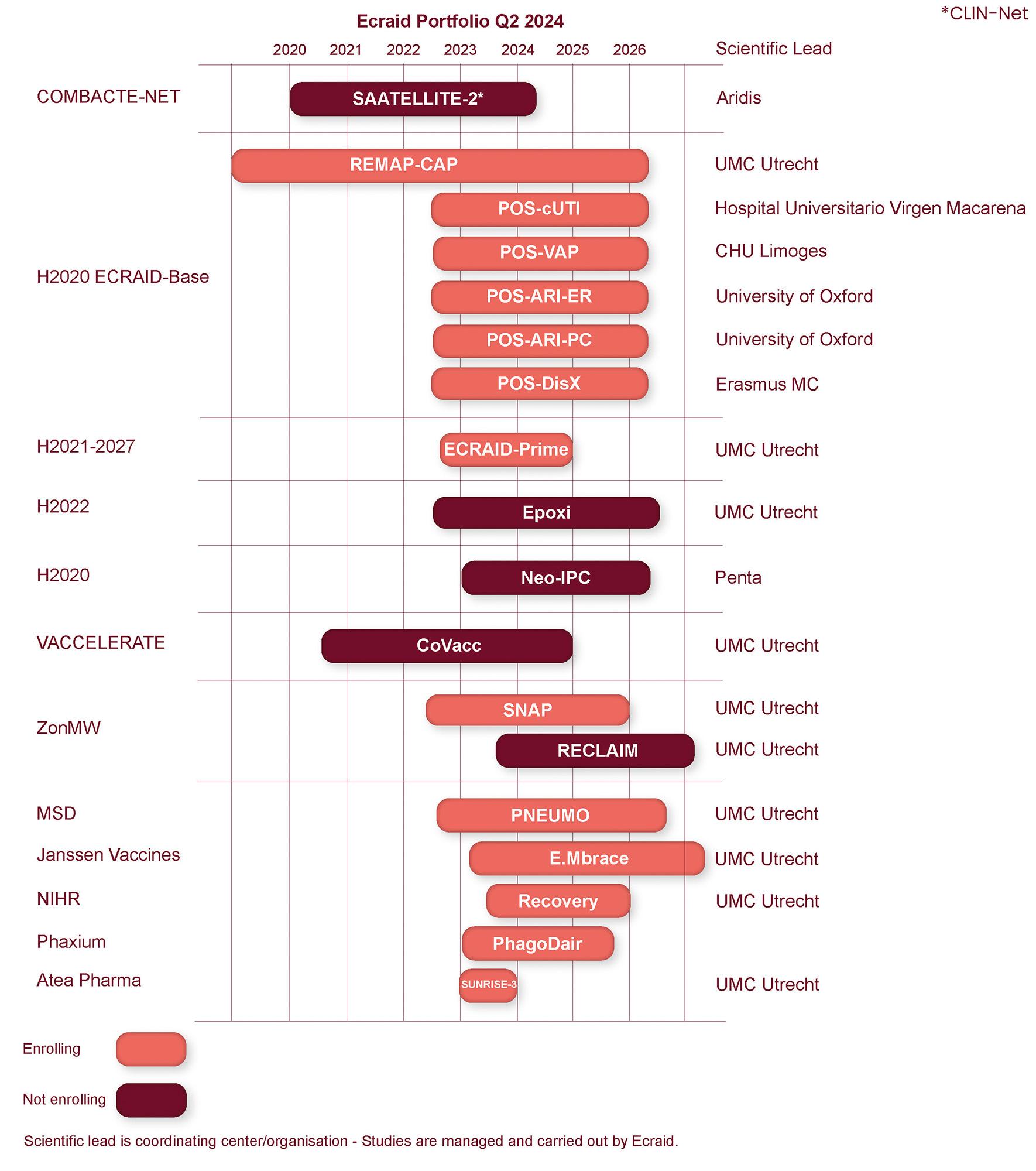
Ecraid portfolio
CoVacc | ECRAID-Prime | E.Mbrace | EPOXI | NeoDeco | PhagoDAIR | PNEUMO | POS-ARI-PC | POS-ARI-ER | POS-cUTI | POS-Disease X | POS-VAP | RECLAIM | RECOVERY | REMAP-CAP | SAATELLITE-2 | SUNRISE-3 | SNAP
CoVacc
Scientific lead: UMC Utrecht
Project manager: Karen van Hulst (karen.vanhulst@ecraid.eu)
Start date: 2020 | End date: 2024
Status: data analyses and CSR
Not seeking partners
ECRAID-Prime
Study name (full):
Scientific lead: UMC Utrecht
Project manager: Ilse Rietveld (ilse.rietveld@ecraid.eu)
Start date: 2021 | End date: perpetual
Status: in preparation
Type of study: adaptive platform trial
Target disease: COVID-19 and COVID-like illnesses
Department: PC
Enrolling: no
Short description: ECRAID-Prime is one of the six projects funded by the European Commission's Horizon Europe program to drive therapeutic and vaccines clinical trials to boost COVID-19 and COVID-like illness treatment and prevention. It will implement Europe's first-of-its-kind adaptive platform trial on COVID-19 therapeutics in the primary care setting.
E.Mbrace
Study name (full):
Scientific lead: UMC Utrecht
Project manager: Andres Scholl (andrea.scholl@ecraid.eu)
Start date: 2022 | End date:
EPOXI
Study name (full): European randomised clinical trial on mPOX Infection
Scientific lead: UMC Utrecht
Project manager: Lina Gurskaite (lina.gurskaite@ecraid.eu)
Start date: 2022 | End date: 2026
Status: in preparation
Type of study: Phase IV randomised controlled double-blind interventional trial
Intervention: tecovirimat
Target disease: mpox
Department: sex health clinics / PC
Target countries: BE, NL, ES, PT, DE, FR, NO, IT
Enrolling: no
Target number of participants: 150
Target population: men who have sex with men, The trial population will consist of adults with laboratory-confirmed mpox; these patients may be (temporarily) hospitalised or treated as out-patients
Inclusion criteria:
1. PCR/NAAT-confirmed mpox infection
2. The presence of active skin or mucosal lesion(s)3
3. Signed Informed Consent Form
Exclusion criteria:
1. Age <18 years.
2. Body weight <40 kg
3. Pregnant and breastfeeding patients are not eligible for inclusion in this study.
4. Lack of mental capacity to provide informed consent
5. Trial participation is considered not in the best interest of patient
6. Previous, current or planned use of another investigational drug 5at any point during study participation.
7. Exclusion criteria for the specific intervention tecovirimat, as described in the protocol.
Short description: Multi-country randomized, placebo-controlled, double-blinded trial to evaluate the safety and efficacy of Tecovirimat SIGA for the treatment of adult patients with mpox disease.
NeoDeco
Study name (full): Optimising kangaroo care to reduce neonatal severe infection/sepsis and resistant bacterial colonisation among high-risk infants in neonatal intensive care: a pragmatic, multicentre, parallel cluster randomised hybrid implementation-effectiveness study.
Scientific lead: Penta
Project manager: Nicolette Van Sluisnico (lette.vansluis@ecraid.eu)
Start date: 2024
Status: in preparation
Type of study: Pragmatic, multicentre, parallel group, cluster randomised hybrid effectiveness-implementation study
Target disease: infection/sepsis and resistant bacterial
Department: Neonatal Intensive Care Unit
Target number of sites: 24
Target countries: 5 (Greece, Switzerland, Spain, Italy and UK)
Enrolling: no
Target number of participants: 2640
Target population: High-risk infants born before 32 weeks’ gestation admitted to neonatal units.
Inclusion criteria: All high-risk infants admitted to participating neonatal units are eligible to contribute to data and sample collection, including non-invasive stool samples, regardless of birth weight, complexity of care, anticipated hospitalisation duration, whether they are admitted to a single room or multi-bed ward and whether they are admitted directly after birth or not.
Exclusion criteria: There will be no exclusion criteria for data or sample collection for high-risk infants admitted to participating neonatal units during the study period. Infants whose parents or legal guardians do not provide written informed consent to data and sample collection will not contribute to the study.
Short description: The overall aim is to investigate the effectiveness of implementation of optimised Kangaroo Care (KC) in line with international best practice recommendations.
PhagoDAIR
Study name (full):
Scientific lead: UMC Groningen
Project manager: Wietske Bouwman (wietske.bouwman@ecraid.eu)
Start date: 2023 | End date:
Download the brochure (in Dutch)
Not seeking partners
PNEUMO
Study name (full):
Scientific lead: UMC Utrecht
Project manager: Tessa Kliest (tessa.kliest@ecraid.eu)
Start date: 2022 | End date:
Status: collecting data; manuscript in preparation
Type of study: observational
Target disease: goal is to understand the risk of specific pneumococcal serotypes
Target number of sites: 51
Target countries: ES, FR, IT, DE, UK and NL
Enrolling: yes
Target number of participants: 8,100
Target population: CAP patients
Short description: PNEUMO is an observational trial recruiting CAP patients with the goal to understand the risk of specific pneumococcal serotypes, their morbidity and mortality to inform vaccine development and public health policy. Exploratory objectives were added to assess the impact of SARS-COV-2 pandemic. MSD acts as funding agent, UMCU is EU Sponsor and the Operational Team is based at Ecraid.
From Ecraid’s network, 51 sites were selected (42 actively recruiting, 2 to be opened, 7 closed) in 6 countries (ES, FR, IT, DE, UK and NL). Recruitment started in FEB-2020 and so far 64% of the global target (over 5700 of 9000) has been included. 62% of the data is locked and the first Manuscript is in preparation. Projections indicate that per end of recruitment (AUG-2025), 8100 inclusions will be reached.
POS-ARI-ER
Study name (full): Perpetual Observational Study on Acute Respiratory Infection in the Emergency Room
Scientific lead: University of Oxford, UK
Project manager: Lina Gurskaite (lina.gurskaite@ecraid.eu)
Start date: 2021 | End date: perpetual
Status: collecting data
Type of study: currently observational; later interventional; will become an adaptive platform trial
Target disease: acute respiratory infection
Department: ER
Target number of sites: 40
Target countries: BE, HR, FR, GR, IT, NL, RO, RS, ES, GB
Enrolling: yes
Target number of participants: 11,750
Target population: Adults (≥ 18 years old) presenting to acute hospital services with suspected
community-acquired acute respiratory tract infection
Inclusion criteria:
1) Age ≥ 18 years
2) Clinical suspicion of a new episode of acute respiratory tract infection, with onset in the last
10 days
3) Patient presents to an emergency room or secondary care setting
4) Informed consent is provided by the patient or their legal representative (or where required
by local regulations, an appropriate Consultee provides a declaration)
Exclusion criteria:
1) Patient has been transferred from another hospital
2) Patient admitted to hospital for >2 days at the time of enrolment
3) Patient has been previously enrolled in the POS-ARI-ER study
Short description: The POS-ARI-ER study is a perpetual, observational study (POS), designed to provide data for clinical characterisation of acute respiratory infections (ARIs) in adults presenting to hospital settings across Europe, and to serve as an overarching research infrastructure for rapid implementation of
randomised controlled trials (RCTs) and other clinical studies related to diagnosis and treatment of
ARI.
POS-ARI-PC
Study name (full): Perpetual Observational Study on Acute Respiratory Infection in Primary Care
Scientific lead: University of Oxford, UK
Project manager: Lina Gurskaite (lina.gurskaite@ecraid.eu)
Start date: 2021 | End date: perpetual
Status: collecting data
Type of study: currently observational; later interventional; will become an adaptive platform trial
Target disease: acute respiratory infection
Department: PC
Target countries: 5-15, BE, GB, FR
Enrolling: yes
Target number of participants: 2,000
Target population: patients presenting with symptoms suggestive of an ARI.
Short description: POS-ARI-PC is a multi-country, prospective Perpetual Observational Study (POS) among patients presenting in PC with symptoms of ARI. It aims to provide an estimate of the overall incidence of illness and individual diagnoses, as well as critically important benchmark descriptive data related to patient characteristics, complications, outcome, and risk factors per viral aetiology. These detailed data allow for the identification of variation in management and care within and between countries, suggesting improvements for care planning and informing clinical guidelines.
POS-cUTI
Study name (full): Perpetual Observational Study on Complicated Urinary Tract Infection
Scientific lead: University Hospital Virgen Macarena, Seville, Spain
Project manager: Lina Gurskaite (lina.gurskaite@ecraid.eu)
Start date: 2021 | End date: perpetual
Status: collecting data
Type of study: currently observational; later interventional; will become an adaptive platform trial
Target disease: complicated urinary tract infection
Department: hospital setting
Target number of sites: 40
Target countries: BE, CY, DK, FR, BG, DE, IT, GR, NL, ES, PT, GB, RO, RS, SE, TR
Enrolling: yes
Target number of participants: 16000
Target population: Patients admitted to participating hospitals with cUTI or who develop a cUTI during their hospital stay.
Inclusion criteria:
1. Age ≥18 years.
2. Patients with complicated urinary infection.
3. Patients with pyelonephritis or bacteremia with a urinary tract source.
Exclusion criteria:
1. Patients with a life expectancy previous to development of cUTI <30 days and those exclusively under palliative care.
2. Patients who died in <48 hours since the presentation with cUTI.
3. Patients participating in RCT for treatment of cUTI.
4. >96 hours since the clinical diagnosis of cUTI.
Short description: POS-cUTI is a multinational, prospective observational cohort study of patients with cUTI to be developed in over 40 hospitals from 10-20 European countries. The primary objective of this study is to provide an infrastructure capable of rapidly implementing randomised controlled trials (RCTs) and other clinical studies related to the treatment of cUTI. The secondary objectives include descriptive analyses related to patient characteristics, complications, outcomes, and risk factors.
POS-Disease X
Study name (full): Perpetual Observational Study on Disease X
Scientific lead: Erasmus MC
Project manager: Lina Gurskaite (lina.gurskaite@ecraid.eu)
Start date: 2021 | End date: perpetual
Status: collecting data
Type of study: observational
Target disease: unexplained febrile illness with unusual clinical presentation and/or epidemiology, and of likely viral aetiology.
Department: ER & other
Target number of sites: 5-8
Target countries: BE, CZ, NL, RS, ES, CH, GB
Enrolling: yes
Target number of participants: 1600
Target population: immunocompromised patients
Short description: This Perpetual Observational Study (POS) in specialised infectious disease hospitals across Europe on severe unexplained febrile illness with unusual epidemiology and/or clinical presentation and of likely virus aetiology (Disease X). This POS serves as a warm-based study that – while addressing specific questions for the current study population- also can be pivoted to include patients in case of an unexpected disease outbreak for which clinical research is needed.
POS-VAP
Study name (full): Perpetual Observational Study on Ventilator-Associated Pneumonia
Scientific lead: CHU Limoges, France
Project manager: Lina Gurskaite (lina.gurskaite@ecraid.eu)
Start date: 2021 | End date: perpetual
Status: collecting data
Type of study: currently observational; later interventional; will become an adaptive platform trial
Target disease: ventilator-associated pneumonia
Department: ICU
Target number of sites: 40
Target countries: Al, BE, HR, CZ, FR, DE, GR, IT, NL, RO, RS, ES, CH, GB
Enrolling: yes
Target number of participants: 20,000
Target population: All patients admitted to an ICU, who are under IMV and documented or expected to be under IMV for more than 48 hours are eligible to participate in the study.
Inclusion criteria:
1. Age ≥18 years
2. At risk of acquiring VAP during ICU stay, defined as: requiring admission or being admitted to the ICU and expected or documented to be under IMV for more than 48 hours.
3. Consent, either a written informed consent given by the study patient in full medical, psychological, cognitive, social or legal capacity to give an informed consent, or, if not possible, by a Legally Authorized Representative of the study patient OR any applicable locally accepted form of consent OR consent waiver allowing data collection and sharing of data according to ECRAID’s principles (see 2.8.3).
Exclusion criteria: Death is deemed to be imminent or inevitable during this hospital admission AND one or more of the patient, substitute decision maker or attending physician are not committed to full active treatment.
Short description: Ventilator-Associated Pneumonia (VAP) is a frequent complication in mechanically-ventilated ICU patients and is often caused by multi-drug resistant bacteria. Distinguishing bacterial colonisation of the respiratory tract from infection of the lung tissue is extremely difficult with currently available diagnostic approaches resulting in an unnecessary use of broad-spectrum antibiotics in many patients, thereby enhancing antimicrobial resistance (AMR). The primary objective of this study is to provide an infrastructure capable of rapidly implementing randomised controlled trials (RCTs) and other clinical trials related to prevention, diagnosis or treatment of VAP in ICUs. The secondary objectives include descriptive analyses related to patient characteristics, complications, outcome and risk factors.
RECLAIM
Study name (full):
Scientific lead: UMC Utrecht
Project manager: Sandra Swart (sandra.swart@ecraid.eu)
Start date: 2023 | End date:
Status: in preparation
Type of study: interventional; adaptive platform trial
Target disease: Long COVID
Department:
Webpage (in Dutch)
Not seeking partners
RECOVERY
Study name (full): Randomised Evaluation of COVID-19 Therapy
Scientific lead: UMC Utrecht
Project manager: Nicolette Van Sluis (nicolette.vansluis@ecraid.eu)
Start date: 2023 | End date:
Status: collecting data
Type of study: Randomized open-label, phase 3 platform trial; adaptive design.
Target disease: CAP and Influenza
Department: Ward and emergency room
Target number of sites: 15 (subject to change)
Target countries: 3 (France, Italy and The Netherlands) (subject to change)
Enrolling: yes
Target number of participants: 500 (30-40 per site)
Target population: Patients with CAP or influenza
Inclusion criteria:
1. Hospitalised aged ≥ 18 years old
2. Pneumonia syndrome:
a) typical symptoms of a new respiratory infection; and
b) objective evidence of acute lung disease (e.g. X-ray or CT, hypoxia, or compatible clinical examination); and
c) alternative causes have been considered unlikely or excluded (e.g. heart failure).
However, the diagnosis remains a clinical one based on the opinion of the managing doctor (the above criteria are just a guide).
3. One of the following diagnoses:
a) Confirmed influenza A or B infection (including patients with SARS-CoV-2 co-infection and/or hospital-acquired infection)
b) CAP with planned antibiotic treatment (excl. patients with suspected or confirmed SARS-CoV-2, influenza, active pulmonary tuberculosis or Pneumocystis jirovecii pneumonia)
4. No medical history that might, in the opinion of the attending clinician, put the patient at significant risk if he/she were to participate in the trial
5. Attending clinician does not believe a specific trial treatment is indicated or contra-indicated
Short description: Better treatments are needed to reduce mortality in patients hospitalised with COVID-19, influenza and CAP. RECOVERY is now evaluating promising treatments (oseltamivir and corticosteroids) for these infections. Currently, 6 study sites (5 French and 1 Dutch) have been activated in Europe. Moreover, 2 French study sites already have their first patients in. 4 patients have been enrolled in the RECOVERY EU trial so far. The Sponsor (University of Oxford) confirmed their interest in including more study sites to the trial. In addition, Baloxavir is planned to be added as a study treatment to the RECOVERY EU trial.
REMAP-CAP
Study name (full): Randomized, Embedded, Multifactorial Adaptive Platform trial for Community-Acquired Pneumonia
Scientific lead: UMC Utrecht
Project manager: Svenja Peters (svenja.peters@ecraid.eu)
Start date: 2014 | End date: perpetual
Status: ongoing
Type of study: interventional; adaptive platform trial
Target disease: community-acquired pneumonia, including influenza CAP and COVID-19
Department: predominantly ICU
Target number of sites: N/A
Target countries: global
Enrolling: yes
Target number of participants: N/A
Target population: adults with severe CAP, including influenza and COVID-19 patients; adults with moderate CAP and children are recruited in the UK only
Inclusion criteria Core Protocol:
1. Adult patient admitted to an ICU for acute severe CAP within 48 hours of hospital admission with
a. symptoms or signs or both that are consistent with lower respiratory tract infection (for example, acute onset of dyspnea, cough, pleuritic chest pain) AND
b. Radiological evidence of new onset infiltrate of infective origin (in patients with pre-existing radiological changes, evidence of new infiltrate)
2. Up to 48 hours after ICU admission, receiving organ support with one or more of:
a. Non-invasive or invasive ventilatory support;
b. Receiving infusion of vasopressor or inotropes or both
Exclusion criteria Core Protocol:
1. Healthcare-associated pneumonia:
a. Prior to this illness, is known to have been an inpatient in any healthcare facility within the last 30 days
b. Resident of a nursing home or long-term care facility
2. Death is deemed to be imminent and inevitable during the next 24 hours AND one or more of the patient, substitute decision maker or attending physician are not committed to full active treatment
3. Previous participation in this REMAP within the last 90 days
There are further eligibility criteria defined for the pandemic appendix, as well as on domain and intervention-level.
Short description: REMAP-CAP is a global adaptive platform trial embedded in clinical care and allowing multiple therapies to be evaluated at the same time. The treatments investigated in the trial are organised in “domains” within which alternative interventions are compared. As such, patients are assigned to regimens consisting of specific interventions within one or more domains.
SAATELLITE-2
Study name (full):
Scientific lead: Aridis
Project manager: Miranda Hopman (miranda.hopman@ecraid.eu)
Start date: January 2020 | End date:
Status: Concluded
Not seeking partners
SUNRISE-3
Study name (full):
Scientific lead: UMC Utrecht
Project manager: Sandra Swart (sandra.swart@ecraid.eu)
Start date: 2023 | End date:
Status: collecting data
Type of study: interventional
SNAP
Study name (full): Staphylococcus aureus Network Adaptive Platform Trial
Scientific lead: UMC Utrecht
Project manager: Janneke Verberk (j.d.m.verberk-2@uncutrecht.nl)
Start date: worldwide beginning of 2022, EU started October 2023
End date: perpetual trial, when funding ends. Note: every EU country have to arrange their own funding for participation.
Status: NL 6 active sites enrolling patients, start-up/preparation other EU countries
Type of study: low-interventional, Randomized, Embedded, Multifactorial Adaptive Platform trial
Target disease: Staphylococcus aureus bacteraemia (SAB)
Department: Infectious diseases
Target number of sites: NA; depending per country and funding
Target countries: NA; as many as possible depending on interest and country funding
Enrolling: yes
Target number of participants: NA
Target population: Patients with Staphylococcus aureus bacteraemia (SAB)
Inclusion criteria:
1. Staphylococcus aureus complex grown from ≥1 blood culture
2. Admitted to participating hospital at anticipated time of eligibility assessment
Exclusion criteria:
1. Time of anticipated platform entry is greater than 72 hours post collection of the index blood culture
2. Polymicrobial bacteraemia, defined as more than one organism (at species level) in the index blood cultures OR in any subsequent blood culture reported between the collection of the index blood culture and platform eligibility assessment, excluding those organisms judged to be contaminants by either the microbiology laboratory or treating clinician
3. Known previous participation in the randomised SNAP platform
4. Known positive blood culture for S. aureus (of the same silo: PSSA, MSSA or MRSA) between 72 hours and 180 days prior to the time of eligibility assessment
5. Treating team deems enrolment in the study is not in the best interest of the patient
6. Treating clinician believes that death is imminent and inevitable
7. Patient is for end-of-life care and antibiotic treatment is considered not appropriate
8. Patient <18 years of age and paediatric recruitment not approved at recruiting site
9. Patient has died since the collection of the index blood culture
Short description: SNAP is an innovative investigator initiated adaptive platform trial with a perpetual aim to identify the effect of a range of clinical interventions on all-cause 90-day mortality in patients with Staphylococcus aureus bacteraemia. The SNAP EU Chief Investigator of this trial is Prof. dr. M.J.M. Bonten from the University Medical Center Utrecht (EU Sponsor; UMCU). Ecraid is coordinating the SNAP trial in the European region; in the Netherlands this is in collaboration with the Radboud UMC. The trial started in 2022 in Australia, initiated by the Global Sponsor University of Melbourne, and has a recruitment period for at least 3 years or longer. Currently, several countries such as Australia, New-Zealand, Canada, Singapore, Israel and UK are participating in this trial with more than 100 sites involved incl. EU.