ECRAID-Prime
European Clinical Research Alliance on Infectious Diseases: Primary care adaptive platform trial for pandemics and epidemics.Study goal
ECRAID-Prime is pleased to announce the addition of a third candidate treatment – LTX-109 intranasal spray – to its adaptive platform trial, which is already assessing the potential benefits of nitric oxide nasal spray (NONS) and saline spray towards improving patient outcomes.
The goal of this Phase 2A study is to evaluate whether LTX-109, produced by Pharma Holdings, accelerates viral pathogens clearance in outpatients with COVID-19 and COVID-like illness, compared with placebo nasal spray.
Current status
Site activations are underway with the first patient expected to be recruited in October 2025. The target for this study, combined with the NONS study, is to enrol a total of 999 patients by the end of 2026. We are committed to the continued evaluation of additional therapeutics over an extended period of time, and will provide further updates as the study progresses.
“LTX-109 is a broad spectrum anti-viral agent with little or no risks of driving antiviral resistance. The molecule originates from Tromsø University and is based on properties of certain proteins found in milk that helps protect the young from infections. So it represents a gentle, but rapidly acting approach that disrupts viruses – an innovation inspired by the best solutions found in nature itself!”
- Chris Butler, ECRAID-Prime co-cooordinating investigator, University of Oxford
Patient Enrolment
[Coming soon]
Participating countries
List of research centres and clinics participating in the ECRAID-Prime LTX-109 study.
| Country | Hospital |
| Belgium | University of Antwerp |
| Ireland | University College Dublin |
| Poland | Medical University of Białystok |
| Spain | IDIAP Jordi Gol - Unitat d'Estudis del Medicament |
| Georgia | Medcapital LLC |

Participant inclusion criteria
- ≥18 years of age.
- Presence of at least 2 symptoms suggestive of COVID-19 or COVID-like illness.
- Illness assessed by the recruiting and medically-qualified clinician or delegate as being due to a respiratory infection.
- Inclusion within 2 days from the onset of symptoms.
- Willing and able to provide informed consent.
- Willing and able to comply with trial procedures.
- For women of child-bearing potential:
• A negative urine pregnancy test.
• Using an effective method of contraception or abstinence for 30 days before and after the days of trial medication administration, or abstinence from heterosexual intercourse.
Participant exclusion criteria
The following participants will be excluded from the study:
- Hospital admission: Requiring hospital admission on the day of screening or inclusion.
- Allergies: Known allergies or hypersensitivities to any of the components used in the formulation of the investigational product or control.
- Precluding conditions: Any disease, condition or disorder that would prevent participation in the trial.
- Upcoming surgery: Any planned major surgery within the next 28 days.
- Concurrent trials: Currently participating in a clinical trial involving an investigational product.
- Study personnel: Any individuals involved in the conduct of the study.
Specifically for ISA D (as for ISA B & C):
- Pregnancy/Breastfeeding: Known to be currently pregnant or breastfeeding.
- Epistaxis and hereditary conditions: Current or history of moderate to severe epistaxis or hereditary haemorrhagic telangiectasia.
- Cerebral spinal fluid leaks: History of cerebral spinal fluid leaks via the sinuses or nose.
- Nasal conditions/surgery: Nasal fracture, nasal tumours, nasal masses, meningoencephalocele, and/or nasal surgery in the past 2 weeks. Any known nasal anatomical anomalies.
How the trial works
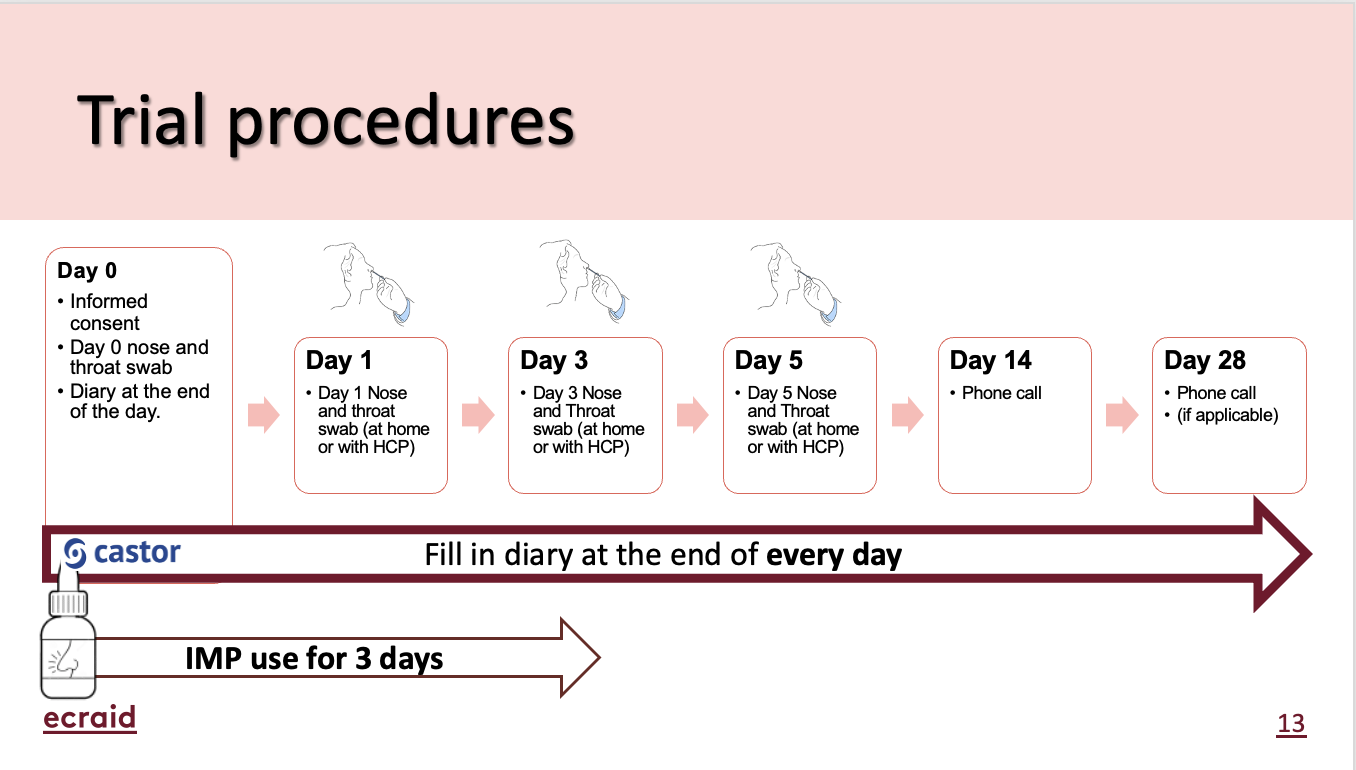
Day 0:
- First clinic visit: Participant’s first visit to the practice
- Informed consent: Doctor or healthcare team obtains and reviews participant's informed consent
- Collect information: Doctor or healthcare team collects participant's baseline information and contact details
- Swabs: Doctor or healthcare team takes a throat/nose swab to determine the cause of participant's respiratory infection.
- Randomisation: Participant is randomly assigned into a study group
- Study supplies: Participant receives study supplies
- Medication begins: Participant begins using medication at home for 5 days
Days 1 – 28:
- Daily diary: Participant records their daily symptoms, impact of symptoms, and healthcare utilisation in their diary.
Days 1, 3 and 5:
- Participants to take a combined throat and nose swab at home.
Day 14:
- Phone call: Short call with the healthcare team to check in on participant's health.
Day 28:
- Contact with participant: To arrange for collection of the swabs and leftover medication.
Unscheduled calls, when necessary:
• Follow-up calls: In the event of (serious) adverse events (SAEs).
About ECRAID-Prime
ECRAID-Prime is one of the six projects funded by the European Commission's Horizon Europe program to drive therapeutic and vaccines clinical trials to boost COVID-19 treatment and prevention. It builds on many years of EU investment in infrastructure for primary care trials and a mature primary care research network that has pioneered novel, efficient, platform clinical trial designs. Read more on www.ecraid.eu/ecraid-prime.
EUCT nr: 2022-501707-27
Contact us
Have a question about the ECRAID-Prime trial or the LTX-109 study?